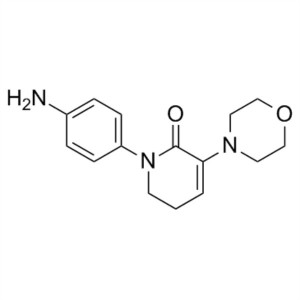
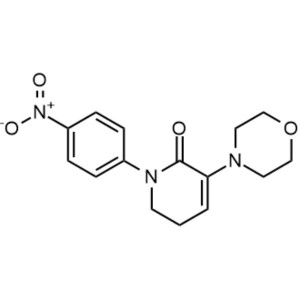
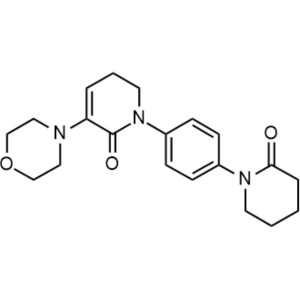
Apixaban CAS 503612-47-3 Purity ≥99.5% (HPLC)
Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer of Apixaban (CAS: 503612-47-3) with high quality. Ruifu Chemical can provide worldwide delivery, competitive price, excellent service, small and bulk quantities available. Purchase Apixaban, Please contact: alvin@ruifuchem.com
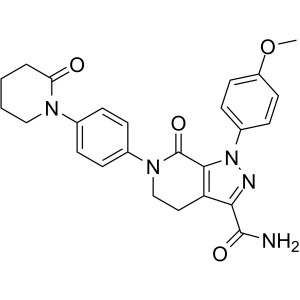
| Chemical Name | Apixaban |
| Synonyms | 1-(4-Methoxyphenyl)-7-oxo-6-[4-(2-oxopiperidin-1-yl)phenyl]-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-c]pyridine-3-carboxamide, 4,5,6,7-Tetrahydro-1-(4-methoxyphenyl)-7-oxo-6-[4-(2-oxo-1-piperidinyl)phenyl]-1H-pyrazolo[3,4-c]pyridine-3-carboxamide, BMS 562247, BMS-562247 |
| Stock Status | In Stock, Commercial Production |
| CAS Number | 503612-47-3 |
| Molecular Formula | C25H25N5O4 |
| Molecular Weight | 459.51 g/mol |
| Melting Point | 235.0~238.0℃ |
| Density | 1.42 |
| COA & MSDS | Available |
| Origin | Shanghai, China |
| Category | API |
| Brand | Ruifu Chemical |
| Items | Inspection Standards | Results |
| Appearance | White to Off-White Crystalline Powder | Complies |
| Loss on Drying | ≤0.50% | 0.10% |
| Residue on Ignition | ≤0.10% | 0.08% |
| Heavy Metals (as Pb) | ≤20ppm | <20ppm |
| Related Substances | ||
| Any Single Impurity | ≤0.50% | Complies |
| Total Impurities | ≤0.50% | Complies |
| Purity / Analysis Method | ≥99.5% (HPLC) | 99.9% |
| Infrared Spectrum | Conforms to Structure | Complies |
| 1 H NMR Spectrum | Conforms to Structure | Complies |
| Conclusion | The product has been tested & complies with the specifications | |
Package: Bottle, Aluminium foil bag, 25kg/Cardboard Drum, or according to customer's requirement.
Storage Condition: Keep the container tightly closed and store in a cool, dry (2~8℃) and well-ventilated warehouse away from incompatible substances. Protect from light and moisture.
Shipping: Deliver to worldwide by air, by FedEx / DHL Express. Provide fast and reliable delivery.
None of the product will be supplied to countries in which this could be in conflict with the existing patents. However the final responsibility lies with the buyer.
For scientific research use only, not for any commercial purpose, not for human or diagnostic use.
How to Purchase? Please contact Dr. Alvin Huang: sales@ruifuchem.com or alvin@ruifuchem.com
15 Years Experience? We have more than 15 years of experience in the manufacture and export of a wide range of high quality pharmaceutical intermediates or fine chemicals.
Main Markets? Sell to domestic market, North America, Europe, India, Korea, Japanese, Australia, etc.
Advantages? Superior quality, affordable price, professional services and technical support, fast delivery.
Quality Assurance? Strict quality control system. Professional equipment for analysis include NMR, LC-MS, GC, HPLC, ICP-MS, UV, IR, OR, K.F, ROI, LOD, MP, Clarity, Solubility, Microbial limit test, etc.
Samples? Most products provide free samples for quality evaluation, shipping cost should be paid by customers.
Factory Audit? Factory audit welcome. Please make an appointment in advance.
MOQ? No MOQ. Small order is acceptable.
Delivery Time? If within stock, three days delivery guaranteed.
Transportation? By Express (FedEx, DHL), by Air, by Sea.
Documents? After sales service: COA, MOA, ROS, MSDS, etc. can be provided.
Custom Synthesis? Can provide custom synthesis services to best fit your research needs.
Payment Terms? Proforma invoice will be sent first after confirmation of order, enclosed our bank information. Payment by T/T (Telex Transfer), PayPal, Western Union, etc.
Apixaban (CAS: 503612-47-3) is a new form of oral anticoagulant drug developed by Bristol Myers Squibb and Pfizer. It is a new form of oral Xa factor inhibitor, and its commercial name is Eliquis. Apixaban is used to treat adult patients undergoing elective hip or knee replacement surgery to prevent venous thromboembolism (VTE)
Apixaban (CAS: 503612-47-3) is an oral selective activated Xa factor inhibitor and can prevent thrombin generation and thrombosis.
Apixaban (CAS: 503612-47-3) is the third new oral anticoagulant to go on sale, following dabigatran and rivaroxaban, and it has already been approved in Europe for preventing venous thromboembolism in patients undergoing elective hip or knee replacement surgery. Out of these three oral anticoagulants approved in Europe, compared to the current standard preventative treatment against venous thromboembolism, enoxaparin, rivaroxaban excelled in the record experiment, and apixaban excelled in the advance experiment. Rivaroxaban’s curative effects were slightly superior, but it caused more severe bleeding than apixaban. Researchers attributed these differences to medication time, as rivaroxaban was taken 6-8 hours after surgery in the record experiment, while apixaban was used 18 hours after surgery in the advance experiment. These drugs have better curative effect when used closer to time of surgery, but also have an increased bleeding risk. Clinical research showed that compared to a daily subdermal injection of 40mg enoxaparin, 2 oral 2.5mg dosages of apixaban had better preventative effects against venous thromboembolism following hip or knee replacement surgery and did not increase bleeding risk.
Apixaban (CAS: 503612-47-3) is a new type of oral Xa factor inhibitor jointly developed by Bristol-Myers Squibb and Pfizer. The trade name is eratol, which is a new type of oral anticoagulant. By inhibiting an important coagulation factor Xa, apixaban can prevent thrombin production and thrombosis. On April 26, 2007, Bristol-Myers Squibb joined hands with Pfizer to announce the cooperation in the development of a new oral anticoagulant apixaban owned by Bristol-Myers Squibb as an upgraded alternative to warfarin. According to the cooperation agreement, Pfizer will pay an advance payment of US $0.25 billion to Bristol-Myers Squibb to bear the 60% of the total development cost of the anticoagulant apixaban (to be implemented from January 1, 2007), while Bristol-Myers Squibb will bear the remaining 40%, thus obtaining the right to jointly develop and sell the drug. In May 2011, apixaban was the first to approve the prevention of venous thrombosis in adult patients undergoing elective hip or knee replacement surgery in 27 EU countries, Iceland and Norway. On November 20, 2012, the European Commission approved Ererto (apixaban) for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF) with one or more risk factors. Subsequently, the Canadian Food and Drug Administration, Japan, and the US FDA approved Ererto? (apixaban) for the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF) with one or more risk factors. On April 12, 2013, the new anticoagulant drug Eloto (ELIQUIS)(apixaban) jointly developed by Bristol-Myers Squibb and Pfizer was officially announced to be listed in China. Ererto is a novel oral factor Xa inhibitor for the prevention of venous thromboembolism (VTE) in adult patients undergoing elective hip or knee replacement. Its listing provides a safe and effective new choice for clinical anticoagulation after orthopedic surgery, and brings good news to Chinese patients undergoing hip/knee elective replacement. Clinical studies have confirmed that compared with 40mg enoxaparin once a day, 2 times a day oral administration of eratol? (apixaban) 2.5mg is more effective for preventing venous thromboembolism after hip or knee replacement surgery, and does not increase the risk of bleeding. Figure 1 shows Elotoapixaban tablets produced by Bristol-Myers Squibb and Pfizer.

![Apixaban Intermediate CAS 27143-07-3 Ethyl Chloro[(4-Methoxyphenyl)hydrazono]acetate Purity ≥99.0% (HPLC)](https://www.ruifuchem.com/uploads/Apixaban-Intermediate-CAS-490-46-0-Factory-Shanghai-Ruifu-Chemical-Co.-Ltd.-www.ruifuchem.com_-300x300.jpg)